Geomembrane chemical compatibility vs. permeability, Part 1
How does permeability occur in an impermeable geomembrane?
We recently concluded a blog series that explored Geomembrane chemical resistance that focused on why it is important. In that series we discussed how to find and interpret chemical resistance data while exploring what that data means. Here, we will take a look at geomembrane chemical compatibility versus permeability.
As a quick review, chemical resistance is one of the most important gateway pieces of data when selecting a geomembrane. But also consider that the barrier properties are assumed to be consistent with chemical resistance. In other words, if a geomembrane is chemically resistant to a fluid–as determined by the steps outlined in the previous blog series−it is also equally suitable as a barrier. But is that true? This series will take a deeper look at that assumption.
We will start with a review of the theory behind permeation through soils and through plastics.
Darcy’s Law
Flow through soil is considered porous flow as water flows around particles. That flow (note it is water, not other liquids) is defined by Darcy’s Law, stated as:
Q = KA (hL/L) or Q = KA (ΔH/ΔL)
Where:
Q = Flow (cm3/sec)
K = Hydraulic Conductivity (cm/sec or cm3/sec/cm2)
ΔH/ΔL = Head loss per flow length (cm/cm)
A = Crosssectional area perpendicular to flow (cm2)
The ΔH/ΔL term is representative of the pressure which drives the liquid through the soil matrix, and is often termed “i”, transforming the equation to: Q = Kai or Q= KiA
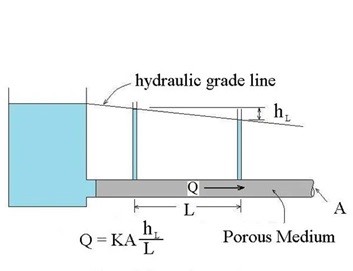 Diagram of Darcy's Law showing Q=KA (hL/L) with hydraulic grade line = hL and Porous Medium.
Diagram of Darcy's Law showing Q=KA (hL/L) with hydraulic grade line = hL and Porous Medium.
Source: Bright Hub Engineering
Hydraulic Conductivity Test: In laboratory, testing for head loss.
The Hydraulic Conductivity, K, is determined by measuring head loss in a laboratory standpipe using a procedure for low permeability (<1 x 10-4 cm/sec) soils, called Falling Head Permeability (ASTM 5084, Standard Test Methods for Measurement of Hydraulic Conductivity of Saturated Porous Material using a Flexible Wall Permeameter). We often refer to K, Hydraulic Conductivity, as “permeability” simply because it represents a rate.

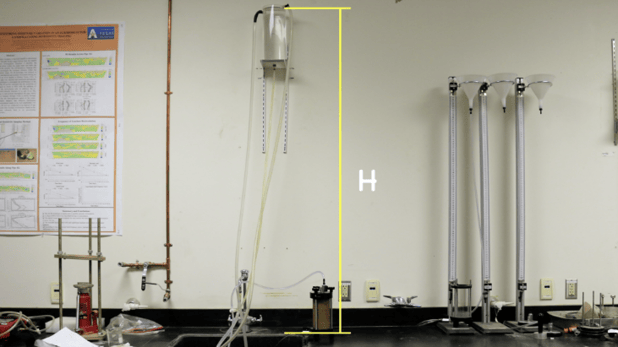 ASTM D5084 laboratory setup showing testing for Falling Head Permeability, lab set up.
ASTM D5084 laboratory setup showing testing for Falling Head Permeability, lab set up.
Source: UT Arlington Pressbooks
Fick’s Law
Plastics are nonporous, and liquids can only pass through by molecular diffusion in and through the plastic, or membrane. Diffusion is described by Fick’s First Law in a steady-state condition (Stark, Choi 2005):
JD = -D (dc/dx)
Where:
JD = Diffusive mass flux, or flow (g/cm2-sec)
-D = Diffusion coefficient, expressed as negative to denote direction of flow (g/cm2/sec)
dc = Change in permeant potential from each side of membrane (g/cm3)
dx = Distance of flow (cm)
On a comparative basis these terms have similar resulting meanings in the two laws:
| Darcy | Fick |
| Q | JD |
| ΔH/ΔL (i) | dc/dx |
| K | D |
D is determined in the laboratory by measuring water or solvent vapor transmission using ASTM D814. More information on this testing can be found here.
 Laboratory permeation cup displayed on laboratory surface, prepping for ASTM D814 testing.
Laboratory permeation cup displayed on laboratory surface, prepping for ASTM D814 testing.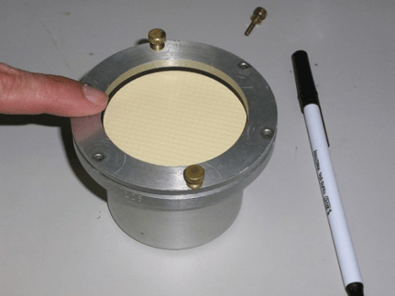 Laboratory permeation cup displayed on laboratory surface, conducting ASTM D814 testing.
Laboratory permeation cup displayed on laboratory surface, conducting ASTM D814 testing.
Source: Seaman Corporation
Permeation of liquids through a polymer membrane, including geomembranes, is a three-step process–with sorption governed by Henry’s Law–which is partial pressure driven and is an equilibrium condition.
Step 1: Sorption. Solution of molecules into the membrane at the side of higher potential governed by Henry’s Law. This is an equilibrium condition.
Step 2: Diffusion. Migration in and through the membrane on a molecular scale per Fick’s Law.
Step 3: Desorption. Release of the diffused molecules on the opposite side of the membrane, Henry’s Law.
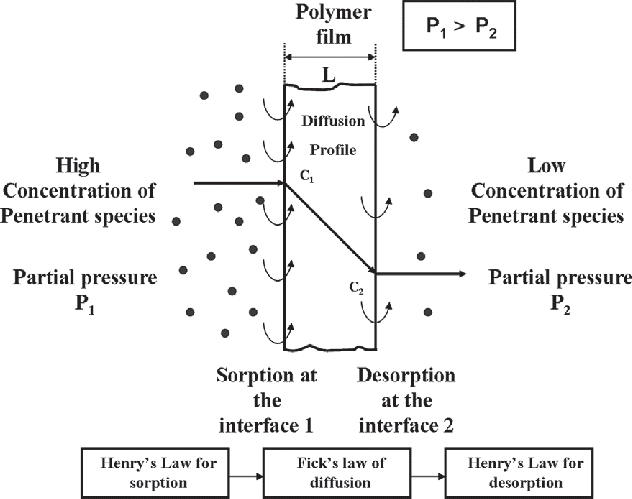 Permeation through Polymer diagram showing steps of Henry's Law sorption to Fick's law of diffusion to Henry's Law for desorption.
Permeation through Polymer diagram showing steps of Henry's Law sorption to Fick's law of diffusion to Henry's Law for desorption.
Source: Ilsoon Lee, Michigan State University
In the geomembrane industry, we routinely reference Fick’s Law as the sole mechanism governing permeation. Diffusion is usually the rate-determining step, but the sorption step (Henry’s Law) is important when evaluating the permeability of a geomembrane, particularly with various chemicals. A liquid must first enter the geomembrane in a molecular form. The nature of the permeant (chemical) in relation to the nature of the membrane has a good deal of influence on the sorption process.
On the other hand, chemical resistance is evaluated by physical changes which are caused by contact with a chemical or liquid. Many, but not all, of the factors influencing permeability also apply to chemical resistance. Simply stated, chemical resistance measures the ability to resist changes to the membrane or polymer that damage its ability to maintain physical properties. A geomembrane may be permeable to a given solute without damaging the polymer.
In our next blog post, we will dig deeper into the factors influencing both permeability and geomembrane chemical resistance.
 An XR-5 geomembrane gas containment cover located in New Hampshire, utilized for dairy process wastewater.
An XR-5 geomembrane gas containment cover located in New Hampshire, utilized for dairy process wastewater.
Source: Geomembrane Technologies Inc.



