Geomembrane chemical compatibility vs. permeability, Part 2
What are the factors affecting permeability of polymers?
In our previous post, How Does Permeability Occur in an Impermeable Geomembrane, we detailed the mechanisms of flow through polymers and explained the governing laws–Fick’s and Henry’s–behind them. Chemical resistance is generally measured by the ability to retain mechanical properties after contact with a given fluid. Likewise, a geomembrane can be chemically attacked in several ways–all of which are related to a solute changing the chemical structure of the polymer–and retain certain properties. Many of the same considerations from our previous discussion apply when we discuss permeability but produce results in a different manner.
Solubility and diffusivity determine the permeability of a polymer. The following are contributing factors. Note that “polymer” will refer to a geomembrane polymer, and “permeant” will refer to a contained liquid.
- Molecular Polarity – Think of this as “like dissolves like.” Polymers and permeants are either polar or non-polar based on the orientation of the base molecules. For example, water is polar, and oil is non-polar. They do not mix. Polar polymers will tend to diffuse polar permeants. The same applies with non-polar polymers and permeants. That does not necessarily guarantee chemical incompatibility but does indicate increased sorption.
- Molecule Size – Small molecules will diffuse at a higher rate compared to larger molecules. Water is a small molecule. Crude oil is considered a complex large molecule. Using fractional distillation, crude oil is separated into smaller molecules for various fuel uses, as displayed below.
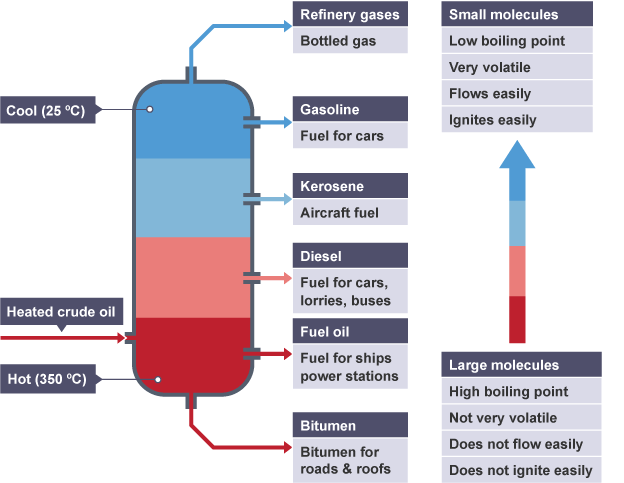 Diagram of fractional distillation of crude oil. Both small molecules and large molecules are named, including: refinery gases, gasoline, kerosene, diesel, fuel oil and bitumen.
Diagram of fractional distillation of crude oil. Both small molecules and large molecules are named, including: refinery gases, gasoline, kerosene, diesel, fuel oil and bitumen.
Source: bbc.co.uk/revisechemistry.uk - Polymer Density – Higher densities provide less area for diffusion.
- Crystallinity – In some polymers, higher crystallinity equals higher density, resulting in increased density. However, some amorphous polymers have much higher densities than crystalline or semicrystalline polymers. Diffusion more readily occurs in amorphous regions, which also exist in semicrystalline polymers. There are completely noncrystalline geomembrane polymers, but few totally crystalline geomembrane polymers due to survivability issues. Instead, semicrystalline is common, possessing both crystalline and amorphous regions. The amorphous regions contain the tie molecules between the crystalline structure that can, under some environmental conditions, be subject to failure.
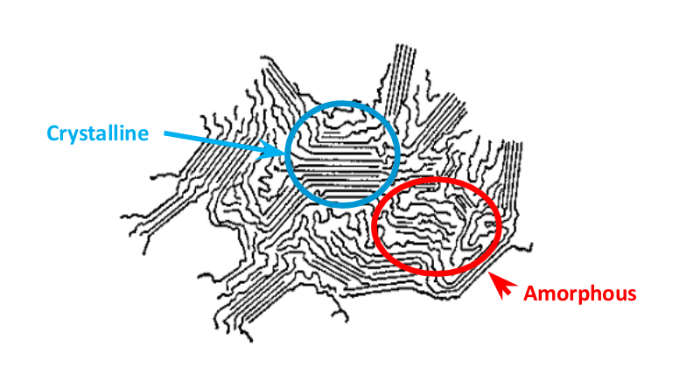 Diagram of a semicrystalline polymer structure, highlighting the crystalline and amorphous portions.
Diagram of a semicrystalline polymer structure, highlighting the crystalline and amorphous portions.
Source: Victrex.com - Differential Pressure – The driving force across the membrane, analogous to hydraulic head in soil permeability.
- Temperature – Higher temperatures increase diffusion rates.
- Thickness – The effect of differential pressure is lessened with increased polymer thickness, which creates a longer path between the higher and lower differential pressure when comparing the same polymers.
Some of these factors influencing permeability also influence chemical resistance, while others do not. As stated earlier, chemical resistance is usually assessed by changes in physical properties such as tensile, elongation, tear and weight after contact with a solute. All those properties can be influenced by:
- Molecular structure of a polymer relative to solute (chemical or solution)
- Chemical concentration
- Exposure Time
- Stress
- Temperature
The base consideration starts with molecular structure. A geomembrane can gain weight or change some other physical property when in contact with a liquid and still maintain physical acceptability. Further, a geomembrane can maintain its physical acceptability in contact with a liquid but have solubility and subsequently high diffusion. In other words, chemical compatibility may not have a parallel relationship with permeability, although we typically assume so.
Take note of what influences geomembrane permeability and chemical resistance. In the next post, we will look at some of those influencers for specific commercial geomembranes.
 Installation of an XR-5 Geomembrane in a Produced Water impoundment. Alaska USA
Installation of an XR-5 Geomembrane in a Produced Water impoundment. Alaska USA
Source: Seaman Corporation



