Geomembrane chemical compatibility vs. permeability, Part 3
How do I use polymer structure data to evaluate geomembrane selection?
In part 2 of our three-part series, we discussed contributing factors to a geomembrane polymer’s permeability. We also explored and explained the five factors influencing chemical resistance. In this concluding post, we look at different geomembranes and the main variables centered around their molecular structure, including density, crystallinity and thickness. Let’s start by noting how various commercial geomembranes vary in these categories.
Molecular Structure (Polarity)
Posts 1 and 2 showed the importance of polarity regarding the liquid to be contained. The following tables outline and expand the Polar/Non-Polar classification of common geomembranes, followed by the Polar/Non-Polar classification of several common solutes.
| Geomembrane Polymer | Geomembrane | Polarity |
| Ethylene Copolymer | XR-5 | Polar |
| HDPE | High-Density Polyethylene | Non-Polar |
| PVC | Polyvinyl Chloride | Polar |
| PP | Polypropylene | Non-Polar |
| LDPE | Low-Density Polyethylene | Non-Polar |
| CSPE | Chlorosulfonated Polyethylene | Polar |
| EPDM | Ethylene Propylene Diene Monomer | Non-Polar |
| Liquid | Polarity |
| Water | Polar |
| Oil | Non-Polar |
| Alcohols | Polar |
| Sulphur Dioxide | Polar |
| Ammonia | Polar |
| Methane | Non-Polar |
| Hydrocarbon Liquids | Non-Polar |
| Cooking Oil | Non-Polar |
Like dissolves like, but geomembranes often contain heterogeneous solutions which could be expected to partition in the sorption process. So, the polarity effect upon permeation may be altered from what is expected with pure solutions.
Density
Solutes travel through openings within a polymer structure. So the denser the structure, the less area or volume is available for transport. The following table gives industry values for specific gravity for the geomembranes listed above. Note: Linear Low-density PE is shown instead of Low-density PE as it is the polymer more commonly used as a geomembrane.
| Geomembrane Polymer | Geomembrane | Density (SG) | Source |
| Ethylene Copolymer | XR-5 | 1.25 min. (reinforced) | Manufacturer spec |
| HDPE | High-Density Polyethylene | 0.94 min. avg | GRI GM 13 |
| PVC | Polypropylene Chloride | 1.2 min. avg | ASTM D7176 |
| PPE | Polypropylene | 0.85 min. avg (reinforced) | GRI GM 18 |
| LLDPE | Linear Low-Density Polyethylene | 0.94 max. | GRI GM 17 |
| CSPE | Chlorosulfonated Polyethylene | 0.85 min. avg. (reinforced) | GRI GM 28 |
| EPDM | Ethylene Propylene Diene Monomer | 1.1-1.19 | Geosynthetics 2020 Spec guide and manufacturer's spec |
Crystallinity
The amount of–or lack of–crystallinity in a geomembrane polymer can influence many performance properties. Higher crystallinity can be expected to produce lower permeability than what lower crystallinity would produce. But some performance properties are negatively impacted with higher crystallinity, including resistance to environmental stress cracking and higher rates of thermal expansion-contraction. For these and other reasons, crystallinity is usually limited. However, permeation occurs primarily in amorphous regions due to random placement of molecules. Amorphous zones typically exist in all polymers including those considered semicrystalline or crystalline. The following table gives a general designation regarding crystallinity for these commercial geomembranes.
| Geomembrane Polymer | Geomembrane | Crystallinity |
| Ethylene Copolymer | XR-5 | Amorphous |
| HDPE | High-Density Polyethylene | Medium to highly crystalline |
| PVC | Polyvinyl Chloride | Amorphous |
| PP | Polypropylene | Amorphous to slightly crystalline |
| LLDPE | Linear Low-Density Polyethylene | Low to semicrystalline |
| CSPE | Chlorosulfonated Polyethylene | Amorphous to slightly crystalline |
| EPDM | Ethylene Propylene Diene Monomer | Amorphous |
Thickness
The longer the flow path, the more differential pressure required for flow, resulting in lower permeability. More polymer is better when comparing like geomembranes, but often irrelevant when comparing different polymers.
| Geomembrane Polymer | Geomembrane | Thickness |
| Ethylene Copolymer | XR-5 | 30-50 mils (0.76-1.3 mm) |
| HDPE | High-Density Polyethylene | 30-120 mils (0.76-3 mm) |
| PVC | Polyvinyl Chloride | 10-60 mils (0.3-1.5 mm) |
| PP | Polypropylene | 30-60 mils (0.76-1.5 mm) |
| LLDPE | Linear Low-Density Polyethylene | 30-120 mils (0.76-3 mm) |
| CSPE | Chlorosulfonated Polyethylene | 36-60 mils (0.9-1.5 mm) |
| EPDM | Ethylene Propylene Diene Monomer | 36-60 mils (0.9-1.5 mm) |
Regarding chemical resistance, the four properties discussed above are in the context of permeability, which is not always proportional to chemical resistance. For instance, if a polymer is thicker, and all other properties remain constant, one would expect lower permeability. However, chemical attack and sorption should not be influenced by thickness.
To illustrate the conclusion of this series, consider two commercial geomembrane products: a reinforced ethylene copolymer (XR-5) and a polyethylene film (HDPE). Both have a manufacturer rating of “A” for containment of jet fuel, but when comparing permeability, the XR-5 tests substantially lower. In fact, the HDPE permeability exceeds U.S. Military standards for collapsible tank storage of JP8, the military equivalent to commercial jet fuel, Jet A.
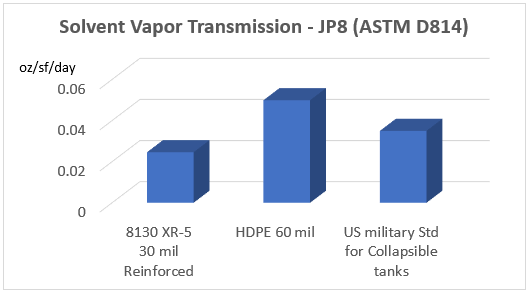
The table highlights solvent vapor transmission of 8130 XR-5 30 mil Reinforced, HDPE 60 mil and U.S. military standard for collapsible tanks.
Source: Seaman Corporation
There are many criteria to consider when evaluating geomembranes; of those, barrier and chemical resistance properties are quite important. Evaluate the polymer, consider the contained liquid, and examine both the anticipated interaction as well as the anticipated performance.

A close-up view of a XR-5 lined jet fuel secondary containment, located in Oregon
Source: ACF West



